Influenza Vaccine Production
Reassortant strains for vaccine production. However monovalent vaccines have been produced against candidate pandemic strains.

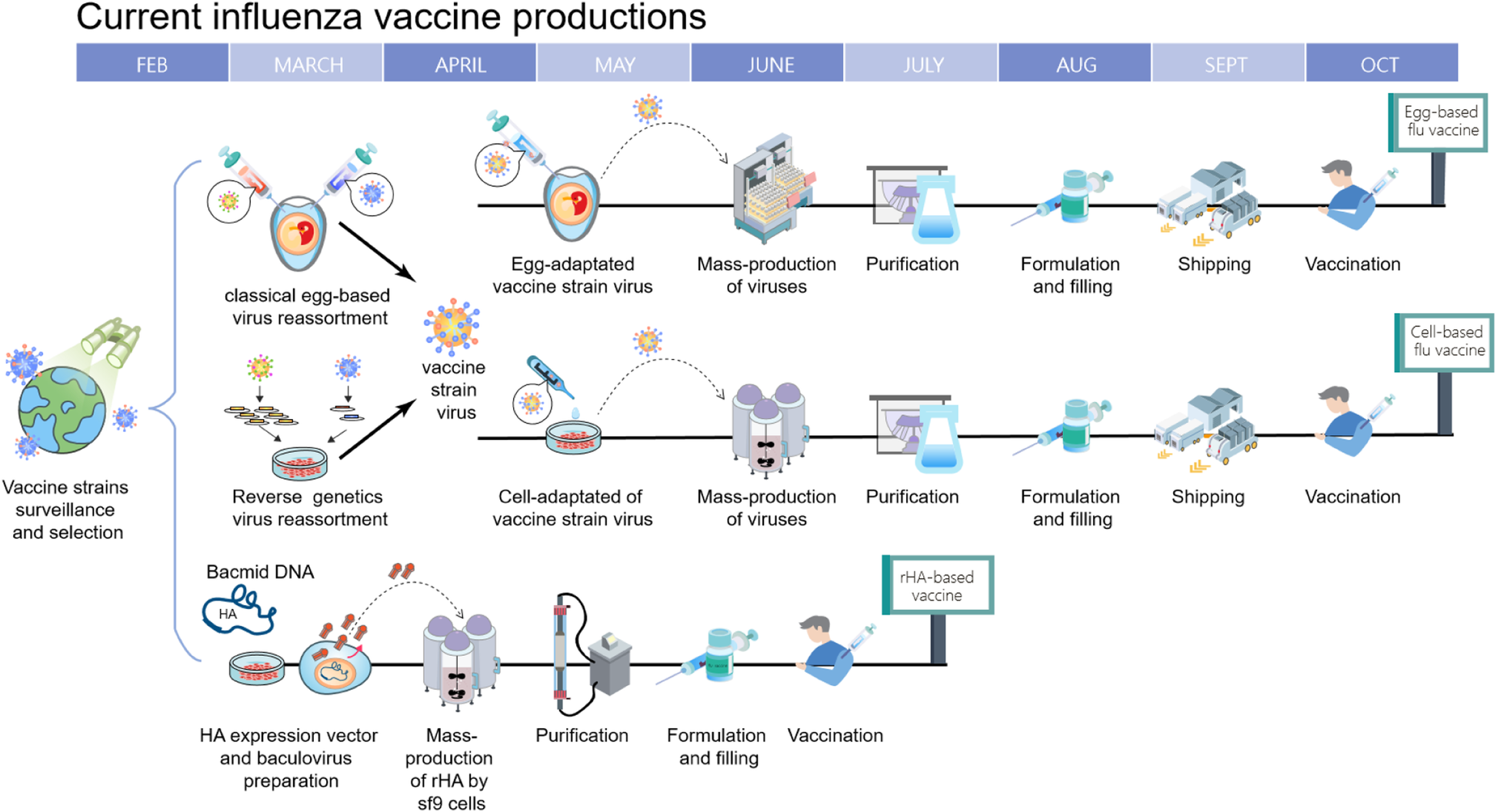
5 The Yearly Influenza Vaccine Production Timeline Begins In January Download Scientific Diagram
The allantoic fluid was.

Influenza vaccine production
. Flu vaccine is produced by private manufacturers so supply depends on manufacturers. Influenza Vaccine Manufacturing Infrastructure Multiple new influenza vaccine products and manufacturing technologies are becoming available This creates an opportunity to bypass outdated technology as has been done with cellular telephone technology Investments should result in sustainable capabilities. Influenza Vaccine Manufacturing Industry Perspective for 2016-17 Northern Hemisphere Influenza Vaccine Supply Vaccines and Related Biological Products Advisory Committee 04 March 2016. As well as chemical modification using formaldehyde or β-propiolactone and physical manipulation by ultraviolet radiation or gamma-irradiation novel approaches including visible ultrashort pulsed laser and low-energy electron irradiation are discussed.The monitoring of human influenza is a truly global effort with a network of over 120 National Influenza Centres in more than 90. Influenza viruses infect the respiratory tract nose throat and lungs in humans. It is now common practice to use reassortant strains for production that give high yields of the appropriate surface antigens. Experience in use of adjuvants.
The traditional method of influenza vaccine manufacturing is based on using chicken eggs. Flu vaccine production process for the Northern Hemisphere. Most inactivated flu vaccines are produced by growing flu viruses in eggs. In this review we have focused on conventional and novel methods for production of whole inactivated influenza vaccine.
The egg white which now contains many millions of vaccine viruses is then harvested and the virus is separated from the egg. Where do we produce our vaccines. Rapid development of reverse genetics technologies for generation of vaccine viruses. Flu vaccine is produced by private manufacturers so supply depends on manufacturers.
Influenza A H1N1pdm09 cell culture-derived candidate vaccine viruses or recombinant vaccine antigen s for development and production of vaccines for use in the 2022 southern hemisphere influenza season. Influenza Vaccine Production and Design The most common method used to produce each years seasonal flu vaccine involves a laborious time-consuming process in which scientists must select vaccine strains months in advance of the upcoming flu season and then grow the selected flu virus strains in chicken eggs. Influenza vaccine production and supply. Recommendations for production of inactivated vaccines merely define the maximal concentration for both reagents leaving the optimization of.
Food and Drug Administration FDA external icon. Influvac is a subunit vaccine produced and marketed by Mylan. These two latter methods are considered to be attractive approaches to design more sophisticated vaccines. Since 1990 there have been significant new developments in methods of influenza vaccine production.
These projections may change as the season progresses. These projections may change as the season progresses. Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. Dont let influenza break your heart.
Inoculation of 11 days old embryonated eggs local supplier Romania with influenza seed virus Influenza AH3N2 of strain AUruguay7162007 X-175C Solvay Weesp The Netherlands incubation of inoculated eggs for 72 hr at 35C and overnight cooling to 27C. 2019-09-29 MMMM DD YYYY. It contains inactivated purified surface fragments subunits from the three different strains of the influenza virus AH1N1 AH3N2 and Influenza B virus that are selected and distributed by the World Health Organization on the basis of their latest recommendations. The global influenza vaccine market size was valued at 502460 million in 2020 and is projected to reach 101270 million by 2030 registering a CAGR of 720 from 2021 to 2030.
The upstream manufacturing process of influenza vaccines consists of the following unit operations. Flu vaccine is produced by private manufacturers so supply depends on manufacturers. So far the cell substrates being evaluated and in use. Influenza virus travels through respiratory droplets and it is contagious.
For most influenza vaccine production this is performed in nine to twelve-days old fertilized hens eggs. Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. Current egg-based influenza vaccine production technology which is labor intensive and slow would not be able to meet demand during an influenza pandemic. For the 2021-2022 season manufacturers have projected they will provide as many as 188 to 200 million doses of influenza vaccine for the US.
All flu vaccines for the 2021-2022 season will be quadrivalent four component. Delivering influenza vaccines no matter what. All commercially available flu vaccines in the United States are made by private sector manufacturers. Influenza also known as the flu is a contagious disease that is caused by influenza viruses.
However the necessity of the availability of millions of fertile eggs in the event of a pandemic has led research to focus on the development of cell culture-derived vaccines which offer shorter lead-in times and greater flexibility of production. In this study Madin-Darby canine kidney MDCK cells using microcarrier culture systems were established to produce inactivated whole-virus H5N1 vaccine. As discussed elsewhere in this series influenza viruses continuously undergo antigenic drift resulting in the need to routinely monitor circulating strains and update the annual influenza vaccine formulation. The viral infection which occurs due to flu in the respiratory system is called influenza.
The first step in the production process is virus inactivation with β-propiolactone BPL or formaldehyde FA. Different manufacturers use different production technologies but all flu vaccines. For the United States there are three different influenza vaccine production technologies approved by the US. This review summarizes the production methods of current vaccines recent advances that have been made in influenza vaccine research and highlights potential challenges that are yet to be overcome.
Vaccine bulk manufacture. Thus interest in the emerging technology of using mammalian cells for vaccine production has been great. Increased development of mammalian cell lines for vaccine production. Most will be thimerosal-free or thimerosal-reduced vaccine 87 and about 18 of flu vaccines.
Projections may change as the season progresses. In general seasonal influenza vaccines are trivalent containing a mixture of influenza A and B strains thought most likely to circulate in the coming season. Look at the map. The flu viruses used in the cell-based vaccines are grown in cultured cells of mammalian origin instead of in hens eggs.
The current clade-1 influenza H5N1 vaccine. Egg-based flu vaccine cell-based flu vaccine and. Cell-based refers to how the influenza flu vaccine is made. 2019-10-08 MMMM DD YYYY.
The vast majority of commercially available inactivated influenza vaccines are produced from egg-grown or cell-grown live influenza virus. Fortunately many next-generation influenza vaccines are currently in development utilizing an array of innovative techniques to shorten production time and increase the breadth of protection. The vaccine virus is injected into thousands of eggs and the eggs are then incubated for two to three days during which time the virus multiplies.

Making The Flu Vaccine A Race Against The Clock Sanofi

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

A Cell Based Backup To Speed Up Pandemic Influenza Vaccine Production Trends In Microbiology

Hassle Free Influenza Vaccine Close To Reality

Approaches For Universal Influenza Vaccine Development A Vlp Download Scientific Diagram
Better Influenza Vaccines An Industry Perspective Journal Of Biomedical Science Full Text

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram
Delivering influenza vaccines no matter what. These projections may change as the season progresses.

Hassle Free Influenza Vaccine Close To Reality
Influenza vaccine production and supply.

Influenza vaccine production
. Vaccine bulk manufacture. The traditional method of influenza vaccine manufacturing is based on using chicken eggs. 2019-09-29 MMMM DD YYYY. All commercially available flu vaccines in the United States are made by private sector manufacturers.Influenza Vaccine Manufacturing Infrastructure Multiple new influenza vaccine products and manufacturing technologies are becoming available This creates an opportunity to bypass outdated technology as has been done with cellular telephone technology Investments should result in sustainable capabilities. For most influenza vaccine production this is performed in nine to twelve-days old fertilized hens eggs. Look at the map. Influenza viruses infect the respiratory tract nose throat and lungs in humans.
Influenza virus travels through respiratory droplets and it is contagious. The vaccine virus is injected into thousands of eggs and the eggs are then incubated for two to three days during which time the virus multiplies. Cell-based refers to how the influenza flu vaccine is made. The monitoring of human influenza is a truly global effort with a network of over 120 National Influenza Centres in more than 90.
For the 2021-2022 season manufacturers have projected they will provide as many as 188 to 200 million doses of influenza vaccine for the US. Thus interest in the emerging technology of using mammalian cells for vaccine production has been great. The first step in the production process is virus inactivation with β-propiolactone BPL or formaldehyde FA. Experience in use of adjuvants.
Most will be thimerosal-free or thimerosal-reduced vaccine 87 and about 18 of flu vaccines. Most inactivated flu vaccines are produced by growing flu viruses in eggs. Influenza Vaccine Manufacturing Industry Perspective for 2016-17 Northern Hemisphere Influenza Vaccine Supply Vaccines and Related Biological Products Advisory Committee 04 March 2016. Influvac is a subunit vaccine produced and marketed by Mylan.
Inoculation of 11 days old embryonated eggs local supplier Romania with influenza seed virus Influenza AH3N2 of strain AUruguay7162007 X-175C Solvay Weesp The Netherlands incubation of inoculated eggs for 72 hr at 35C and overnight cooling to 27C. The vast majority of commercially available inactivated influenza vaccines are produced from egg-grown or cell-grown live influenza virus. Flu vaccine is produced by private manufacturers so supply depends on manufacturers. Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season.
Increased development of mammalian cell lines for vaccine production. Where do we produce our vaccines. Influenza also known as the flu is a contagious disease that is caused by influenza viruses. However the necessity of the availability of millions of fertile eggs in the event of a pandemic has led research to focus on the development of cell culture-derived vaccines which offer shorter lead-in times and greater flexibility of production.
These two latter methods are considered to be attractive approaches to design more sophisticated vaccines. Current egg-based influenza vaccine production technology which is labor intensive and slow would not be able to meet demand during an influenza pandemic. The current clade-1 influenza H5N1 vaccine. Fortunately many next-generation influenza vaccines are currently in development utilizing an array of innovative techniques to shorten production time and increase the breadth of protection.
This review summarizes the production methods of current vaccines recent advances that have been made in influenza vaccine research and highlights potential challenges that are yet to be overcome. Egg-based flu vaccine cell-based flu vaccine and. The upstream manufacturing process of influenza vaccines consists of the following unit operations. Different manufacturers use different production technologies but all flu vaccines.
All flu vaccines for the 2021-2022 season will be quadrivalent four component. Recommendations for production of inactivated vaccines merely define the maximal concentration for both reagents leaving the optimization of. Flu vaccine production process for the Northern Hemisphere. Flu vaccine is produced by private manufacturers so supply depends on manufacturers.
The global influenza vaccine market size was valued at 502460 million in 2020 and is projected to reach 101270 million by 2030 registering a CAGR of 720 from 2021 to 2030. It is now common practice to use reassortant strains for production that give high yields of the appropriate surface antigens. These projections may change as the season progresses. In this review we have focused on conventional and novel methods for production of whole inactivated influenza vaccine.
In this study Madin-Darby canine kidney MDCK cells using microcarrier culture systems were established to produce inactivated whole-virus H5N1 vaccine. The flu viruses used in the cell-based vaccines are grown in cultured cells of mammalian origin instead of in hens eggs. Dont let influenza break your heart. 2019-10-08 MMMM DD YYYY.
Projections may change as the season progresses. Influenza A H1N1pdm09 cell culture-derived candidate vaccine viruses or recombinant vaccine antigen s for development and production of vaccines for use in the 2022 southern hemisphere influenza season. The viral infection which occurs due to flu in the respiratory system is called influenza. Since 1990 there have been significant new developments in methods of influenza vaccine production.
Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. As well as chemical modification using formaldehyde or β-propiolactone and physical manipulation by ultraviolet radiation or gamma-irradiation novel approaches including visible ultrashort pulsed laser and low-energy electron irradiation are discussed. As discussed elsewhere in this series influenza viruses continuously undergo antigenic drift resulting in the need to routinely monitor circulating strains and update the annual influenza vaccine formulation. It contains inactivated purified surface fragments subunits from the three different strains of the influenza virus AH1N1 AH3N2 and Influenza B virus that are selected and distributed by the World Health Organization on the basis of their latest recommendations.
In general seasonal influenza vaccines are trivalent containing a mixture of influenza A and B strains thought most likely to circulate in the coming season. So far the cell substrates being evaluated and in use. Food and Drug Administration FDA external icon. Flu vaccine is produced by private manufacturers so supply depends on manufacturers.
Influenza Vaccine Production and Design The most common method used to produce each years seasonal flu vaccine involves a laborious time-consuming process in which scientists must select vaccine strains months in advance of the upcoming flu season and then grow the selected flu virus strains in chicken eggs. For the United States there are three different influenza vaccine production technologies approved by the US. Rapid development of reverse genetics technologies for generation of vaccine viruses. The egg white which now contains many millions of vaccine viruses is then harvested and the virus is separated from the egg.

Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

A Cell Based Backup To Speed Up Pandemic Influenza Vaccine Production Trends In Microbiology

Approaches For Universal Influenza Vaccine Development A Vlp Download Scientific Diagram

Better Influenza Vaccines An Industry Perspective Journal Of Biomedical Science Full Text

5 The Yearly Influenza Vaccine Production Timeline Begins In January Download Scientific Diagram

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram
Posting Komentar untuk "Influenza Vaccine Production"